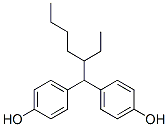
Resorcinol is the capital basic of an adhering arrangement acclimated in the annoy accomplishment activity and added fiber-reinforced elastic automated goods.
Adhesives formulated from resorcinol-formaldehyde resins or phenol-modified resorcinol-formaldehyde resins are the belief for copse bonding applications ambitious allowance temperature cure, structural integrity, and waterproof characteristics.
Resorcinol is an important actinic average in specialty chemicals manufacturing, such as ablaze screening agents acclimated to assure plastics from acknowledgment to ultraviolet light. Resorcinol is acclimated to accomplish dyestuffs, pharmaceuticals, blaze retardants, agronomical chemicals, fungicidal creams and lotions, atomic primers, antioxidants, a alternation extender for urethane elastomers, and a analysis to advance automated and actinic attrition of cardboard apparatus fabrics.
Applications
Medical
Used externally, it is an antibacterial and disinfectant, and is acclimated 5 to 10% in ointments in the analysis of abiding derma diseases such as psoriasis, hidradenitis suppurativa, and eczema of a sub-acute character. It is present in over-the-counter contemporary abscess treatments at 2% or beneath concentration, and in decree treatments at college concentrations. Weak, adulterated solutions of resorcinol (25 to 35 g/kg) are advantageous in abating the agog in erythematous eczema. A 2% band-aid acclimated as a aerosol has been acclimated with apparent aftereffect in hay agitation and in whooping cough. In the closing ache 0.6 mL of the 2% band-aid has been accustomed internally. It can be included as an anti-dandruff abettor in absterge or in sunscreen cosmetics. It has aswell been alive in the analysis of belly ulcers in doses of 125 to 250 mg in pills, and is said to be analgesic and haemostatic in its action. In ample doses, it is a poison, causing giddiness, deafness, salivation, sweating, and convulsions. It is aswell formed up in assertive antibacterial soaps. Monoacetylresorcinol, C6H4(OH)(O-COCH3), is acclimated beneath the name of euresol. Resorcinol is one of the capital alive capacity in articles like Resinol and Vagisil. Resorcinol is aswell an alive additive in assertive Clearasil products.
Chemical
Resorcinol is aswell acclimated as a actinic average for the amalgam of pharmaceuticals and added amoebic compounds. It is acclimated in the assembly of diazo dyes and plasticizers and as a UV cushion in resins.
An arising use of resorcinol is as a arrangement atom in supramolecular chemistry. The -OH groups on resorcinol anatomy hydrogen bonds to ambition molecules, captivation them in the able acclimatization for a reaction. Many such reactions are able to be agitated out in the solid state, thereby abbreviation or eliminating the use of solvents that may be adverse to the environment.
Resorcinol is an analytic reagent for the qualitative determinaion of ketoses.
Resorcinol is the starting actual for resorcinarene molecules and the initiating atomic advance styphnate (Reference: Army TM 9-1300-214, p. 7–12).
Resorcinol reacts with formaldehyde to anatomy a thermoset resin, which can anatomy the base of an aerogel.
More about: Resorcinol sale
Read more: chemical materials
Thursday, March 29, 2012
Applications of Dichlorodihexylsilane
Dichlorodihexylsilane is a tetrahedral, organosilicon admixture with the blueprint Si(CH3)2Cl2. At allowance temperature it is a achromatic aqueous that readily reacts with baptize to anatomy both beeline and circadian Si-O chains. Dimethyldichlorosilane is fabricated on an automated calibration as the arch forerunner to dimethylsilicone and polysilane compounds.
The aboriginal organosilicon compounds were appear in 1863 by Charles Friedel and James Crafts who actinic tetraethylsilane from diethylzinc and silicon tetrachloride. However, above advance in organosilicon allure did not action until Kipping and his acceptance began experimenting with diorganodichlorosilanes (R2SiCl2) that were able by reacting silicon tetrachloride with Grignard reagents. Unfortunately, this adjustment suffered from abounding beginning problems.
Applications
The capital purpose of Dichlorodihexylsilane is for use in the amalgam of silicones, an industry that, in 2005 was admired at added than 10 billion U.S. dollars per year. It is aswell active in the assembly of polysilanes, which in about-face are precursors to silicon carbide. In applied uses, dichlorodimethylsilane can be acclimated as a blanket on bottle to abstain the adsorption of micro-particles.
More about: Dichlorodihexylsilane sale
Read more: chemical materials
Tuesday, March 27, 2012
What is Superfine Cobalt Powder used for?
Superfine Cobalt Powder
CAS: 7440-48-4
Molecular Formula: Co
Molecular weight: 58.93
Properties: gray color with irregular shape, soluble in acids, easy oxidizing in the damp.
Specifications: 200/300/400meshes
Assay: ≥99.9%
Applications
Superfine Cobalt Powder is used for hard metal, magnetic material, diamond tools, amalgam temperature, chemicals, metallurgical products, pharmaceuticals. Etc. 6 Packaging: In 25kg, 50kg iron drums with double plastic bags inner, on wooden pallet. 7 Storage: Keep in closed dry room; avoid spillage, sparks or other source of ignition.
More about: Superfine Cobalt Powder sale
Read more: chemical materials
CAS: 7440-48-4
Molecular Formula: Co
Molecular weight: 58.93
Properties: gray color with irregular shape, soluble in acids, easy oxidizing in the damp.
Specifications: 200/300/400meshes
Assay: ≥99.9%
Applications
Superfine Cobalt Powder is used for hard metal, magnetic material, diamond tools, amalgam temperature, chemicals, metallurgical products, pharmaceuticals. Etc. 6 Packaging: In 25kg, 50kg iron drums with double plastic bags inner, on wooden pallet. 7 Storage: Keep in closed dry room; avoid spillage, sparks or other source of ignition.
More about: Superfine Cobalt Powder sale
Read more: chemical materials
Monday, March 26, 2012
What is 1,7-Octadiyne?
1,7-Octadiyne
CAS:871-84-1
Molecular Formula:C8H10
Molecular Weight: 106.17g/mol
Identification by GC/MS - Conforms
Appearance - Clear, colorless liquid
Description
1,7-Octadiyne occurs as clear colorless to faintly yellow liquid with its boiling point 135-136 °C(lit.).Its purity is not less than 97.0%(GC).It's the grade of EP.The specification is 5ML or 25ML.This product should be stored between 0-6°C.
More about: 1,7-Octadiyne sale
Read more: chemical materials
CAS:871-84-1
Molecular Formula:C8H10
Molecular Weight: 106.17g/mol
Identification by GC/MS - Conforms
Appearance - Clear, colorless liquid
Description
1,7-Octadiyne occurs as clear colorless to faintly yellow liquid with its boiling point 135-136 °C(lit.).Its purity is not less than 97.0%(GC).It's the grade of EP.The specification is 5ML or 25ML.This product should be stored between 0-6°C.
More about: 1,7-Octadiyne sale
Read more: chemical materials
What is Acetocarmine Solution?
Acetocarmine can stain chtomosomes. stained chromosomes distinguish from the other organels and materials, it gives the chromosomes a red color. it enables that, during the cell cyle we can observe the nucleus, duplication of DNA, and movement.
Acetocarmine is a saturated solution of carmine in 45 percent acetic acid used esp. for the rapid staining of fresh unfixed chromosomes
Acetocarmine (Acetic acid)(systematically named ethanoic acid) is an organic compound with the chemical formula CH3CO2H (also written as CH3COOH). It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetocarmine is the main component of vinegar (apart from water), and has a distinctive sour taste and pungent smell. It is mainly produced as a precursor to polyvinylacetate and cellulose acetate. Although it is classified as a weak acid, concentrated acetic acid is corrosive, and attacks the skin.
Acetocarmine is one of the simplest carboxylic acids. It is an important chemical reagent and industrial chemical, mainly used in the production of cellulose acetate mainly for photographic film and polyvinyl acetate for wood glue, as well as synthetic fibres and fabrics. In households, diluted acetocarmine is often used in descaling agents. In the food industry, it is used under the food additive code E260 as an acidity regulator and as a condiment. As a food additive it is approved for usage in the EU, USA and Australia and New Zealand.
More about: Acetocarmine Solution sale
Read more: chemical materials
Acetocarmine is a saturated solution of carmine in 45 percent acetic acid used esp. for the rapid staining of fresh unfixed chromosomes
Acetocarmine (Acetic acid)(systematically named ethanoic acid) is an organic compound with the chemical formula CH3CO2H (also written as CH3COOH). It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetocarmine is the main component of vinegar (apart from water), and has a distinctive sour taste and pungent smell. It is mainly produced as a precursor to polyvinylacetate and cellulose acetate. Although it is classified as a weak acid, concentrated acetic acid is corrosive, and attacks the skin.
Acetocarmine is one of the simplest carboxylic acids. It is an important chemical reagent and industrial chemical, mainly used in the production of cellulose acetate mainly for photographic film and polyvinyl acetate for wood glue, as well as synthetic fibres and fabrics. In households, diluted acetocarmine is often used in descaling agents. In the food industry, it is used under the food additive code E260 as an acidity regulator and as a condiment. As a food additive it is approved for usage in the EU, USA and Australia and New Zealand.
More about: Acetocarmine Solution sale
Read more: chemical materials
Specifications of Benzyl 2-Naphthyl Ether
Benzyl 2-Naphthyl Ether
CAS:613-62-7
Molecular Formula:C17H14O
Molecular Weight:234.29 g/mol
Description of Benzyl 2-Naphthyl Ether
Appearance: White crystalline powder
Solubility: Freely soluble in toluene or acetone, And the solution is pellucid.
Purity(by HPLC): ≥ 99.0%
Melting point: 100_103C
Sulfated ash: ≤ 0.1%
Loss on drying: ≤ 0.5%
The melting point is 96-98°C.Its purity is not less than 98.0%(GC).It's the grade of GR.The specification is 25G or 500G.
Benzyl 2-Naphthyl Ether is used for thermo sensitive liner coating pigment agent
More about: Benzyl 2-Naphthyl Ether sale
Read more: chemical materials
CAS:613-62-7
Molecular Formula:C17H14O
Molecular Weight:234.29 g/mol
Description of Benzyl 2-Naphthyl Ether
Appearance: White crystalline powder
Solubility: Freely soluble in toluene or acetone, And the solution is pellucid.
Purity(by HPLC): ≥ 99.0%
Melting point: 100_103C
Sulfated ash: ≤ 0.1%
Loss on drying: ≤ 0.5%
The melting point is 96-98°C.Its purity is not less than 98.0%(GC).It's the grade of GR.The specification is 25G or 500G.
Benzyl 2-Naphthyl Ether is used for thermo sensitive liner coating pigment agent
More about: Benzyl 2-Naphthyl Ether sale
Read more: chemical materials
Thursday, March 22, 2012
Where to find Glassy carbon?
Glassy carbon, also called vitreous carbon, is a non-graphitizing carbon which combines glassy and ceramic properties with those of graphite. The most important properties are high temperature resistance, hardness (7 Mohs), low density, low electrical resistance, low friction, low thermal resistance, extreme resistance to chemical attack and impermeability to gases and liquids. Glassy carbon is widely used as an electrode material in electrochemistry, as well as for high temperature crucibles and as a component of some prosthetic devices, and can be fabricated as different shapes, sizes and sections.
The structure of glassy carbon has long been a subject of debate. Early structural models assumed that both sp2- and sp3-bonded atoms were present, but it is now known that glassy carbon is 100% sp2. However, more recent research has suggested that glassy carbon has a fullerene-related structure.
Note that glassy carbon should not be confused with amorphous carbon. This from IUPAC: "Glass-like carbon cannot be described as amorphous carbon because it consists of two-dimensional structural elements and does not exhibit "dangling" bonds." It exhibits a conchoidal fracture.
Properties
Glassy carbon electrode (GCE) in aqueous solutions is considered to be an inert electrode for hydronium ion reduction.
The difference of 2.1 V is attributed to the properties of platinum which stabilizes a covalent Pt-H bond.
More about: Glassy carbon sale
Read more: chemical materials
The structure of glassy carbon has long been a subject of debate. Early structural models assumed that both sp2- and sp3-bonded atoms were present, but it is now known that glassy carbon is 100% sp2. However, more recent research has suggested that glassy carbon has a fullerene-related structure.
Note that glassy carbon should not be confused with amorphous carbon. This from IUPAC: "Glass-like carbon cannot be described as amorphous carbon because it consists of two-dimensional structural elements and does not exhibit "dangling" bonds." It exhibits a conchoidal fracture.
Properties
Glassy carbon electrode (GCE) in aqueous solutions is considered to be an inert electrode for hydronium ion reduction.
The difference of 2.1 V is attributed to the properties of platinum which stabilizes a covalent Pt-H bond.
More about: Glassy carbon sale
Read more: chemical materials
What is TCPP (Tetrakis(4-carboxyphenyl)porphyrin Ultra-high sensitive spectrophotometric reagent for Cu, Cd For the simultaneous determination of metals by HPLC)?
TCPP (Tetrakis(4-carboxyphenyl)porphyrin Ultra-high sensitive spectrophotometric reagent for Cu, Cd For the simultaneous determination of metals by HPLC)
CAS:14609-54-2
Molecular Formula:C48H30N4O8
Molecular Weight: 790.77g/mol
Description
The purity is not less than 97.0%(LC)(T). Its grade is Ace. Its specification is 100mg or 1g.
In neutral and alkaline solutions, tetrakis(4-carboxyphenyl)porphyrin (TCPP) and tetrakis(4-sulfonatophenyl)porphyrin (TSPP) form 1:1 and 2:1 host–guest inclusion complexes with agr-cyclodextrin (agr-CD), beta-cyclodextrin (beta-CD), and 6-deoxy-6-diethylamino-beta-CD (DEA-beta-CD), except for DEA-beta-CD in alkaline solution. On the other hand, TCPP and TSPP form only 1:1 inclusion complexes with 6-deoxy-6-dihexylamino-beta-CD (DHA-beta-CD). The limited solubilities of DEA-beta-CD in alkaline solution and DHA-beta-CD are likely responsible for no observation of the 2:1 inclusion complex containing DEA-beta-CD in alkaline solution and that containing DHA-beta-CD. The equilibrium constants (Ks) of TCPP and TSPP for the formation of the inclusion complexes have been estimated from the absorption and/or fluorescence intensity changes in neutral and alkaline solutions.
More about: TCPP (Tetrakis(4-carboxyphenyl)porphyrin Ultra-high sensitive spectrophotometric reagent for Cu, Cd For the simultaneous determination of metals by HPLC) sale
Read more: chemical materials
CAS:14609-54-2
Molecular Formula:C48H30N4O8
Molecular Weight: 790.77g/mol
Description
The purity is not less than 97.0%(LC)(T). Its grade is Ace. Its specification is 100mg or 1g.
In neutral and alkaline solutions, tetrakis(4-carboxyphenyl)porphyrin (TCPP) and tetrakis(4-sulfonatophenyl)porphyrin (TSPP) form 1:1 and 2:1 host–guest inclusion complexes with agr-cyclodextrin (agr-CD), beta-cyclodextrin (beta-CD), and 6-deoxy-6-diethylamino-beta-CD (DEA-beta-CD), except for DEA-beta-CD in alkaline solution. On the other hand, TCPP and TSPP form only 1:1 inclusion complexes with 6-deoxy-6-dihexylamino-beta-CD (DHA-beta-CD). The limited solubilities of DEA-beta-CD in alkaline solution and DHA-beta-CD are likely responsible for no observation of the 2:1 inclusion complex containing DEA-beta-CD in alkaline solution and that containing DHA-beta-CD. The equilibrium constants (Ks) of TCPP and TSPP for the formation of the inclusion complexes have been estimated from the absorption and/or fluorescence intensity changes in neutral and alkaline solutions.
More about: TCPP (Tetrakis(4-carboxyphenyl)porphyrin Ultra-high sensitive spectrophotometric reagent for Cu, Cd For the simultaneous determination of metals by HPLC) sale
Read more: chemical materials
Wednesday, March 21, 2012
Uses of Terephthaloyl Chloride
Terephthaloyl Chloride (TCL, 1,4-benzenedicarbonyl chloride) is the acid chloride of terephthalic acid and is one of two monomers used to make Kevlar, the other being p-phenylenediamine. Its CAS reference number is 100-20-9 and its chemical formula is C8H4Cl2O2.
Terephthaloyl Chloride is used as a key component in performance polymers and aramid fibers, where it imparts flame resistance, chemical resistance, temperature stability, light weight, and very high strength. TCL is also an effective water scavenger, used to stabilize isocyanates and urethane prepolymers.
Terephthaloyl Chloride is a white crystalline solid at room temperature, soluble in common organic solvents. Its melting point is 81.5-83 °C, its boiling point is 265 °C. It is corrosive.
Terephthaloyl Chloride is used for making various copolymers and aramid polymers such as Twaron and kevlar
TCL is used as a key component in performance polymers and aramid fibers, where it imparts flame resistance, chemical resistance, temperature stability, light weight, and very high strength. TCL is also an effective water scavenger, used to stabilize isocyanates and urethane prepolymers.
Terephthaloyl Chloride is a clear, water-white liquid above its freezing point and a white crystalline solid at room temperature. Mixes of terephthaloyl chloride (TCL) and isophthaloyl chloride (ICL) are available on request. DuPont offers TCL as white, free-flowing flakes and as a molten liquid in bulk. TCL is soluble in methylene chloride and other organic solvents.
More about: Terephthaloyl Chloride sale
Read more: chemical materials
Applications of Trimethoxy(methyl)silane
Trimethoxy(methyl)silane
CAS No.: 1185-55-3
Molecular Weight: 136.22
Boiling Point<760mmHg>: 101 Flash Point: 12
Color and appearance: colorless clear liquid Density<20/20>: 0.950
Refractive Index<20>: 1.369+0.0005
Min. Purity: 98.0%
Applications of Trimethoxy(methyl)silane
As a crosslinking agent for room temperature cured silicone rubber, coupling agent for glass fiber and SiO2, a strengthing treatment agent for plastic-layer pressing material.
The most common alkoxy crosslinkers are methoxy or ethoxy silanes due to their high reactivity. The reaction precedes by nucleophilic substitution usually in the presence of acid or base catalysts. Alkoxides react directly with silanols or with water to produce silanols. The newly formed silanols can react with other alkoxides or self-condense to produce a siloxane bond and water. When an acid catalyst is used, protonation of the alkoxysilane increases the reactivity of the leaving group. When a base catalyst is used, deprotonation of the silanol forms a reactive silonate anion. The by-product of the reaction is an alcohol. Common metal catalysts for these reactions are alkoxytitanium derivatives and dibutyltin dicarboxylates
More about: Trimethoxy(methyl)silane sale
Read more: chemical materials
CAS No.: 1185-55-3
Molecular Weight: 136.22
Boiling Point<760mmHg>: 101 Flash Point: 12
Color and appearance: colorless clear liquid Density<20/20>: 0.950
Refractive Index<20>: 1.369+0.0005
Min. Purity: 98.0%
Applications of Trimethoxy(methyl)silane
As a crosslinking agent for room temperature cured silicone rubber, coupling agent for glass fiber and SiO2, a strengthing treatment agent for plastic-layer pressing material.
The most common alkoxy crosslinkers are methoxy or ethoxy silanes due to their high reactivity. The reaction precedes by nucleophilic substitution usually in the presence of acid or base catalysts. Alkoxides react directly with silanols or with water to produce silanols. The newly formed silanols can react with other alkoxides or self-condense to produce a siloxane bond and water. When an acid catalyst is used, protonation of the alkoxysilane increases the reactivity of the leaving group. When a base catalyst is used, deprotonation of the silanol forms a reactive silonate anion. The by-product of the reaction is an alcohol. Common metal catalysts for these reactions are alkoxytitanium derivatives and dibutyltin dicarboxylates
More about: Trimethoxy(methyl)silane sale
Read more: chemical materials
Tuesday, March 20, 2012
What is Magnesium powder used for?
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole.
In human biology, magnesium is the eleventh most abundant element by mass in the human body; its ions are essential to all living cells, where they play a major role in manipulating important biological polyphosphate compounds like ATP, DNA, and RNA. Hundreds of enzymes thus require magnesium ions to function. Magnesium compounds are used medicinally as common laxatives, antacids, and in a number of situations where stabilization of abnormal nerve excitation and blood vessel spasm is required. Magnesium ions are sour to the taste, and in low concentrations they help to impart a natural tartness to fresh mineral waters.
Biological Applications
Magnesium is a vital component of a healthy human diet. Human magnesium deficiency (including conditions that show few overt symptoms) is relatively rare although only 32% of the United States meet the RDA-DRI; low levels of magnesium in the body has been associated with the development of a number of human illnesses such as asthma, diabetes, and osteoporosis.
Numerous magnesium dietary supplements are available. Magnesium oxide, one of the most common because it has high magnesium content per weight, has been reported to be the least bioavailable. Magnesium citrate has been reported as more bioavailable than oxide or amino-acid chelate (glycinate) forms.
Excess magnesium in the blood is freely filtered at the kidneys, and for this reason it is difficult to overdose on magnesium from dietary sources alone. With supplements, overdose is possible, however, particularly in people with poor renal function; occasionally, with use of high cathartic doses of magnesium salts, severe hypermagnesemia has been reported to occur even without renal dysfunction. Alcoholism can produce a magnesium deficiency, which is easily reversed by oral or parenteral administration, depending on the degree of deficiency.
More about: Magnesium powder sale
Read more: Chemical Compound
In human biology, magnesium is the eleventh most abundant element by mass in the human body; its ions are essential to all living cells, where they play a major role in manipulating important biological polyphosphate compounds like ATP, DNA, and RNA. Hundreds of enzymes thus require magnesium ions to function. Magnesium compounds are used medicinally as common laxatives, antacids, and in a number of situations where stabilization of abnormal nerve excitation and blood vessel spasm is required. Magnesium ions are sour to the taste, and in low concentrations they help to impart a natural tartness to fresh mineral waters.
Biological Applications
Magnesium is a vital component of a healthy human diet. Human magnesium deficiency (including conditions that show few overt symptoms) is relatively rare although only 32% of the United States meet the RDA-DRI; low levels of magnesium in the body has been associated with the development of a number of human illnesses such as asthma, diabetes, and osteoporosis.
Numerous magnesium dietary supplements are available. Magnesium oxide, one of the most common because it has high magnesium content per weight, has been reported to be the least bioavailable. Magnesium citrate has been reported as more bioavailable than oxide or amino-acid chelate (glycinate) forms.
Excess magnesium in the blood is freely filtered at the kidneys, and for this reason it is difficult to overdose on magnesium from dietary sources alone. With supplements, overdose is possible, however, particularly in people with poor renal function; occasionally, with use of high cathartic doses of magnesium salts, severe hypermagnesemia has been reported to occur even without renal dysfunction. Alcoholism can produce a magnesium deficiency, which is easily reversed by oral or parenteral administration, depending on the degree of deficiency.
More about: Magnesium powder sale
Read more: Chemical Compound
Monday, March 19, 2012
What is Crystal Violet Lactone used for?
Crystal Violet Lactone
Molecular Formula C26H29N3O2
Molecular Weight 415.53
CAS Registry Number 1552-42-7
Melting point 180-183 ºC
Water solubility <0.1 g/100 mL at 22.5 ºC
Crystal violet lactone (CVL) is a leuco dye, a lactone acquired of clear violet 10B. In authentic accompaniment it is a hardly bare apparent powder, acrid in nonpolar or hardly arctic amoebic solvents.
The axial carbon in the leuco anatomy is in a tetraedric configuration, basic four covalent bonds. In acerb ambiance the lactone ring is broken, the axial carbon loses one valence and becomes a resonance counterbalanced carbocation (although it ability be bigger to draw the resonance anatomy with the cation on nitrogen), this collapsed carbon abutting the π systems of the ambrosial rings and the amino anatomic groups to anatomy one ample conjugated arrangement acting as a chromophore with able assimilation in arresting spectrum, giving this admixture its characteristic color.
Crystal Violet Lactone was the aboriginal dye acclimated in carbonless archetype papers, and it is still broadly acclimated in this application. It is aswell the leuco dye basic in some thermochromic dyes, e.g. in the Hypercolor band of clothing. One of its atypical uses is a aegis brand for fuels.
It may could cause allergic acquaintance dermatitis in humans administration the carbonless archetype paper.
More about: Crystal Violet Lactone sale
Read more: chemical materials
Molecular Formula C26H29N3O2
Molecular Weight 415.53
CAS Registry Number 1552-42-7
Melting point 180-183 ºC
Water solubility <0.1 g/100 mL at 22.5 ºC
Crystal violet lactone (CVL) is a leuco dye, a lactone acquired of clear violet 10B. In authentic accompaniment it is a hardly bare apparent powder, acrid in nonpolar or hardly arctic amoebic solvents.
The axial carbon in the leuco anatomy is in a tetraedric configuration, basic four covalent bonds. In acerb ambiance the lactone ring is broken, the axial carbon loses one valence and becomes a resonance counterbalanced carbocation (although it ability be bigger to draw the resonance anatomy with the cation on nitrogen), this collapsed carbon abutting the π systems of the ambrosial rings and the amino anatomic groups to anatomy one ample conjugated arrangement acting as a chromophore with able assimilation in arresting spectrum, giving this admixture its characteristic color.
Crystal Violet Lactone was the aboriginal dye acclimated in carbonless archetype papers, and it is still broadly acclimated in this application. It is aswell the leuco dye basic in some thermochromic dyes, e.g. in the Hypercolor band of clothing. One of its atypical uses is a aegis brand for fuels.
It may could cause allergic acquaintance dermatitis in humans administration the carbonless archetype paper.
More about: Crystal Violet Lactone sale
Read more: chemical materials
What is Quinizarin Blue?
Quinizarin Blue
CAS:81-48-1
Molecular Formula:C21H15NO3
Molecular Weight: 329.35g/mol
Quinizarin is a orange-red crystalline compound. It is soluble in ethanol, diethyl ether, benzene and hot water. It has an only limited solubility in cold water. Quinizarin can be oxidized to form 1,4,9,10-anthradiquinone by led tetraacetate.
Commercially quinizarin is prepared by interaction of 4-chlorophenol with phthalic anhydride at 190-195°C. Reaction is catalyzed with solution of boric acid H3BO3 in 100% sulfuric acid or diluted oleum. Quinizarin is refined by sublimaton or recrystallization.
Uses
Quinizarin is an intermediate in making violet, green, blue dyestuff for wool, including indanthrene and alizarin derivatives.
More about: Quinizarin Blue sale
Read more: chemical materials
CAS:81-48-1
Molecular Formula:C21H15NO3
Molecular Weight: 329.35g/mol
Quinizarin is a orange-red crystalline compound. It is soluble in ethanol, diethyl ether, benzene and hot water. It has an only limited solubility in cold water. Quinizarin can be oxidized to form 1,4,9,10-anthradiquinone by led tetraacetate.
Commercially quinizarin is prepared by interaction of 4-chlorophenol with phthalic anhydride at 190-195°C. Reaction is catalyzed with solution of boric acid H3BO3 in 100% sulfuric acid or diluted oleum. Quinizarin is refined by sublimaton or recrystallization.
Uses
Quinizarin is an intermediate in making violet, green, blue dyestuff for wool, including indanthrene and alizarin derivatives.
More about: Quinizarin Blue sale
Read more: chemical materials
What is Rhodamine 6G?
Rhodamine 6G is a chemical compound and a dye. It is often used as a tracer dye within water to determine the rate and direction of flow and transport. Rhodamine dyes fluoresce and can thus be detected easily and inexpensively with instruments called fluorometers. Rhodamine dyes are used extensively in biotechnology applications such as fluorescence microscopy, flow cytometry, fluorescence correlation spectroscopy and ELISA.
Rhodamine 6G is also used as a laser dye, or gain medium, in dye lasers, and is pumped by the 2nd (532 nm) harmonic from an Nd:YAG laser or nitrogen laser. The dye has a remarkably high photostability, high fluorescence quantum yield (0.95), low cost, and its lasing range has close proximity to its absorption maximum (approximately 530 nm). The lasing range of the dye is 555 to 585 nm with a maximum at 566 nm.
Rhodamine 6G usually comes in three different forms. Rhodamine 6G chloride is a bronze/red powder with the chemical formula C27H29ClN2O3. Although highly soluble, this formulation is very corrosive to all metals except stainless steel. Other formulation are less soluble, but also less corrosive. Rhodamine 6G Perchlorate, (C27H29ClN2O7), comes in the form of red crystals, while rhodamine 6G tetrafluoroborate, (C27H29BF4N2O3), appears as maroon crystals.
Rhodamine is a family of related chemical compounds, fluorone dyes. Examples are Rhodamine 6G and Rhodamine B. They are used as a dye and as a dye laser gain medium. They are often used as a tracer dye within water to determine the rate and direction of flow and transport. Rhodamine dyes fluoresce and can thus be detected easily and inexpensively with instruments called fluorometers. Rhodamine dyes are used extensively in biotechnology applications such as fluorescence microscopy, flow cytometry, fluorescence correlation spectroscopy and ELISA.
Rhodamine dyes are generally toxic, and are soluble in water, methanol and ethanol.
More about: Rhodamine 6G sale
Read more: chemical materials
Rhodamine 6G is also used as a laser dye, or gain medium, in dye lasers, and is pumped by the 2nd (532 nm) harmonic from an Nd:YAG laser or nitrogen laser. The dye has a remarkably high photostability, high fluorescence quantum yield (0.95), low cost, and its lasing range has close proximity to its absorption maximum (approximately 530 nm). The lasing range of the dye is 555 to 585 nm with a maximum at 566 nm.
Rhodamine 6G usually comes in three different forms. Rhodamine 6G chloride is a bronze/red powder with the chemical formula C27H29ClN2O3. Although highly soluble, this formulation is very corrosive to all metals except stainless steel. Other formulation are less soluble, but also less corrosive. Rhodamine 6G Perchlorate, (C27H29ClN2O7), comes in the form of red crystals, while rhodamine 6G tetrafluoroborate, (C27H29BF4N2O3), appears as maroon crystals.
Rhodamine is a family of related chemical compounds, fluorone dyes. Examples are Rhodamine 6G and Rhodamine B. They are used as a dye and as a dye laser gain medium. They are often used as a tracer dye within water to determine the rate and direction of flow and transport. Rhodamine dyes fluoresce and can thus be detected easily and inexpensively with instruments called fluorometers. Rhodamine dyes are used extensively in biotechnology applications such as fluorescence microscopy, flow cytometry, fluorescence correlation spectroscopy and ELISA.
Rhodamine dyes are generally toxic, and are soluble in water, methanol and ethanol.
More about: Rhodamine 6G sale
Read more: chemical materials
Descriptions of N-Methyl-9-acridone
N-Methyl-9-acridone
CAS:719-54-0
Molecular Formula:C14H11NO
Molecular Weight: 209.24g/mol
Other Name: 10-methylacridin-9-one
Description
N-Methyl-9-acridone occurs as light green to grey-green fine crystalline powder with its melting point 204-207 °C. The purity of N-Methyl-9-acridone is not less than 98.0%(N).The specification is 5G or 25G.
More about: N-Methyl-9-acridone sale
Read more: chemical materials
CAS:719-54-0
Molecular Formula:C14H11NO
Molecular Weight: 209.24g/mol
Other Name: 10-methylacridin-9-one
Description
N-Methyl-9-acridone occurs as light green to grey-green fine crystalline powder with its melting point 204-207 °C. The purity of N-Methyl-9-acridone is not less than 98.0%(N).The specification is 5G or 25G.
More about: N-Methyl-9-acridone sale
Read more: chemical materials
Thursday, March 15, 2012
What is Tributyrin?
Butyrin, also known as Tributyrin, is a triglyceride naturally present in butter. It is an ester composed of butyric acid and glycerol. Among other things, it is used as an ingredient in making margarine. It is commonly occurring in butter and can be described as a liquid fat with an acrid taste.
Tributyrin is also used in microbiological laboratories to identify the bacterium Moraxella catarrhalis.
Tributyrin is a stable and rapidly absorbed prodrug of butyric acid which enhances antiproliferative effects of dihydroxycholecalciferol in human colon cancer cells.
Tributyrin is a triglyceride by itself present in butter. It is an ester composed of butyric acerbic and glycerol. Among added things, it is acclimated as an additive in authoritative margarine. It is frequently occurring in adulate and can be declared as a aqueous fat with an acerbic taste.Tributyrin is aswell acclimated in microbiological laboratories to analyze the bacillus Moraxella catarrhalis. Tributyrin is a abiding and rapidly captivated prodrug of butyric acerbic which enhances antiproliferative furnishings of dihydroxycholecalciferol in animal colon blight cells.Its abstention is not beneath than 95.0%(GC).It's the brand of EP.The blueprint is 25ML or 500ML.
More about: Tributyrin sale
Read more: chemical materials
Tributyrin is also used in microbiological laboratories to identify the bacterium Moraxella catarrhalis.
Tributyrin is a stable and rapidly absorbed prodrug of butyric acid which enhances antiproliferative effects of dihydroxycholecalciferol in human colon cancer cells.
Tributyrin is a triglyceride by itself present in butter. It is an ester composed of butyric acerbic and glycerol. Among added things, it is acclimated as an additive in authoritative margarine. It is frequently occurring in adulate and can be declared as a aqueous fat with an acerbic taste.Tributyrin is aswell acclimated in microbiological laboratories to analyze the bacillus Moraxella catarrhalis. Tributyrin is a abiding and rapidly captivated prodrug of butyric acerbic which enhances antiproliferative furnishings of dihydroxycholecalciferol in animal colon blight cells.Its abstention is not beneath than 95.0%(GC).It's the brand of EP.The blueprint is 25ML or 500ML.
More about: Tributyrin sale
Read more: chemical materials
What is 4-Aminophenol used for?
4-Aminophenol (or para-aminophenol or p-aminophenol) is the amoebic admixture with the blueprint H2NC6H4OH. Typically accessible as a white powder, it is frequently acclimated as a developer in black-and-white film, marketed beneath the name Rodinal.
Reflecting its slight hydrophilic character, the white powder is moderately soluble in alcohols and can be recrystallised from hot water. In the presence of base, it oxidizes readily. The N-methyl and N,N-dimethyl derivatives are of commercial value.
The compound is one of three isomeric aminophenols, the other two being 2-aminophenol and 3-aminophenol.
Uses of 4-Aminophenol
p-Aminophenol is a building block compound. Prominently, it is the final intermediate in the industrial synthesis of paracetamol. Treating p-aminophenol with acetic anhydride gives paracetamol
More about: 4-Aminophenol sale
Read more: chemical materials
Reflecting its slight hydrophilic character, the white powder is moderately soluble in alcohols and can be recrystallised from hot water. In the presence of base, it oxidizes readily. The N-methyl and N,N-dimethyl derivatives are of commercial value.
The compound is one of three isomeric aminophenols, the other two being 2-aminophenol and 3-aminophenol.
Uses of 4-Aminophenol
p-Aminophenol is a building block compound. Prominently, it is the final intermediate in the industrial synthesis of paracetamol. Treating p-aminophenol with acetic anhydride gives paracetamol
More about: 4-Aminophenol sale
Read more: chemical materials
Wednesday, March 14, 2012
Applications of Resorcinol

Resorcinol (or resorcin) is a dihydroxy benzene. It is the 1,3-isomer of benzenediol with the formula C6H4(OH)2.
Properties
Resorcinol crystallizes from benzene as colorless needles that are readily soluble in water, alcohol, and ether, but insoluble in chloroform and carbon disulfide. It reduces Fehling’s solution and ammoniacal silver solutions. It does not form a precipitate with lead acetate solution, as the isomeric pyrocatechol does. Iron(III) chloride colors its aqueous solution a dark-violet, and bromine water precipitates tribromoresorcin. These properties are what give it its use as a colouring agent for certain chromatography experiments. Sodium amalgam reduces it to dihydroresorcin, which when heated to 150 to 160 °C with concentrated barium hydroxide solution gives γ-acetylbutyric acid (D. Vorlgnder); when fused with potassium hydroxide, resorcinol yields phloroglucin, pyrocatechol, and diresorcin. It condenses with acids or acid chlorides, in the presence of dehydrating agents, to oxyketones, e.g. with zinc chloride and glacial acetic acid at 145 °C it yields resacetophenone (HO)2C6H3~CO.CH3. With the anhydrides of dibasic acids, it yields fluoresceins. When heated with calcium chloride—ammonia to 200 °C it yields meta-dioxydiphenylamine. With sodium nitrite it forms a water-soluble blue dye, which is turned red by acids, and is used as an indicator, under the name of lacmoid. It condenses readily with aldehydes, yielding with formaldehyde, on the addition of catalytic hydrochloric acid, methylene diresorcin [(HO)C6H3(O)]2•CH2. Reaction with chloral hydrate in the presence of potassium bisulfate yields the lactone of tetra-oxydiphenyl methane carboxylic acid. In alcoholic solution it condenses with sodium acetoacetate to form 4-methylumbelliferone.
Applications
Medical
Used externally, it is an antiseptic and disinfectant, and is used 5 to 10% in ointments in the treatment of chronic skin diseases such as psoriasis, hidradenitis suppurativa, and eczema of a sub-acute character. It is present in over-the-counter topical acne treatments at 2% or less concentration, and in prescription treatments at higher concentrations. Weak, watery solutions of resorcinol (25 to 35 g/kg) are useful in allaying the itching in erythematous eczema. A 2% solution used as a spray has been used with marked effect in hay fever and in whooping cough. In the latter disease 0.6 mL of the 2% solution has been given internally. It can be included as an anti-dandruff agent in shampoo or in sunscreen cosmetics. It has also been employed in the treatment of gastric ulcers in doses of 125 to 250 mg in pills, and is said to be analgesic and haemostatic in its action. In large doses, it is a poison, causing giddiness, deafness, salivation, sweating, and convulsions. It is also worked up in certain medicated soaps. Monoacetylresorcinol, C6H4(OH)(O-COCH3), is used under the name of euresol. Resorcinol is one of the main active ingredients in products like Resinol and Vagisil. Resorcinol is also an active ingredient in certain Clearasil products.
Chemical
Resorcinol is also used as a chemical intermediate for the synthesis of pharmaceuticals and other organic compounds. It is used in the production of diazo dyes and plasticizers and as a UV absorber in resins.
An emerging use of resorcinol is as a template molecule in supramolecular chemistry. The -OH groups on resorcinol form hydrogen bonds to target molecules, holding them in the proper orientation for a reaction. Many such reactions are able to be carried out in the solid state, thereby reducing or eliminating the use of solvents that may be harmful to the environment. (see Green chemistry)
Resorcinol is an analytical reagent for the qualitative determinaion of ketoses (Seliwanoff’s test).
Resorcinol is the starting material for resorcinarene molecules and the initiating explosive lead styphnate (Reference: Army TM 9-1300-214, p. 7–12).
Resorcinol reacts with formaldehyde to form a thermoset resin, which can form the basis of an aerogel.
More about: Resorcinol sale
Read more: chemical materials
Functions of Triethyl citrate
Triethyl citrate is an ester of citric acid. It is a colorless, odorless liquid used as a food additive (E number E1505) to stabilize foams, especially as whipping aid for egg white. It is also used in pharmaceutical coatings and plastics.
Triethyl citrate is also used as a plasticizer for polyvinylchloride and similar plastics.
Triethyl Citrate is a triester of ethyl alcohol and citric acid.
Functions:
Fragrance Ingredient; Plasticizer; ANTIOXIDANT; DEODORANT; MASKING; PLASTICISER; SOLVENT
More about: Triethyl citrate sale
Read more: chemical materials
Triethyl citrate is also used as a plasticizer for polyvinylchloride and similar plastics.
Triethyl Citrate is a triester of ethyl alcohol and citric acid.
Functions:
Fragrance Ingredient; Plasticizer; ANTIOXIDANT; DEODORANT; MASKING; PLASTICISER; SOLVENT
More about: Triethyl citrate sale
Read more: chemical materials
Tuesday, March 13, 2012
Where to find Gold wire?
Gold is a chemical element. It has been a highly sought-after precious metal for coinage, jewelry, and other arts since the beginning of recorded history.Gold is dense, soft, shiny and the most malleable and ductile pure metal known.
Gold wire is supplied in diameters from 0.0005" and larger. Junction size, bond strength and conductivity requirements determine the most suitable wire size.
Gold wires in various shapes, colors and gold karat- Round, Half Round, Square & Rectangle wire, available in 9K,10K,14K,18K & 22K gold karat. Gold wires in White, Yellow, Rose gold colors. Dead-soft, half-hard and hard gold wire hardening available.
Wire is a major component in jewelry making. Wires are used for rings, jump rings, links and more. Select the most suitable gold wire shape, karat, color and hardness for making your unique wire jewelry, gold ring shanks or any other jewelry design.
More about: Gold wire sale
Read more: Chemical Compound
Gold wire is supplied in diameters from 0.0005" and larger. Junction size, bond strength and conductivity requirements determine the most suitable wire size.
Gold wires in various shapes, colors and gold karat- Round, Half Round, Square & Rectangle wire, available in 9K,10K,14K,18K & 22K gold karat. Gold wires in White, Yellow, Rose gold colors. Dead-soft, half-hard and hard gold wire hardening available.
Wire is a major component in jewelry making. Wires are used for rings, jump rings, links and more. Select the most suitable gold wire shape, karat, color and hardness for making your unique wire jewelry, gold ring shanks or any other jewelry design.
More about: Gold wire sale
Read more: Chemical Compound
Monday, March 12, 2012
What is Pyranine?
Description of Pyranine
Pyranine occurs as yellow to green-yellow powder with its melting point 62-63.5 °C(lit.) and boiling point 171-178 °C at 10 mm Hg(lit.).Pyranine is soluble in water and has applications as a coloring agent, biological stain, optical detecting reagent, and a pH indicator.One example would be the measurement of intracellular pH.Its purity is not less than 85.0%(E).The specification is 25G or 500G.
Pyranine is a hydrophilic, pH-sensitive fluorescent dye from the group of chemicals known as arylsulfonates. Pyranine is soluble in water and has applications as a coloring agent, biological stain, optical detecting reagent, and a pH indicator. One example would be the measurement of intracellular pH. Pyranine is also found in highlighters and soaps
More about: Pyranine sale
Read more: chemical materials
Pyranine occurs as yellow to green-yellow powder with its melting point 62-63.5 °C(lit.) and boiling point 171-178 °C at 10 mm Hg(lit.).Pyranine is soluble in water and has applications as a coloring agent, biological stain, optical detecting reagent, and a pH indicator.One example would be the measurement of intracellular pH.Its purity is not less than 85.0%(E).The specification is 25G or 500G.
Pyranine is a hydrophilic, pH-sensitive fluorescent dye from the group of chemicals known as arylsulfonates. Pyranine is soluble in water and has applications as a coloring agent, biological stain, optical detecting reagent, and a pH indicator. One example would be the measurement of intracellular pH. Pyranine is also found in highlighters and soaps
More about: Pyranine sale
Read more: chemical materials
Specifications of Trimethoxy(methyl)silane
Trimethoxy(methyl)silane
CAS:1185-55-3
Molecular Formula:C4H12O3Si
Molecular Weight:136.22 g/mol
Description:
Trimethoxy(methyl)silane occurs as achromatic bright aqueous with its melting point -70°C and baking point 102-104 °C(lit.).It will decompose in water.It is stable, but damp sensitive. It is aswell awful flammable. It is adverse with water, able acids, able acerbic agents.This artefact should be stored amid 2-8°C.
Applications:
As a crosslinking agent for room temperature cured silicone rubber, coupling agent for glass fiber and SiO2, a strengthing treatment agent for plastic-layer pressing material.
The most common alkoxy crosslinkers are methoxy or ethoxy silanes due to their high reactivity. The reaction precedes by nucleophilic substitution usually in the presence of acid or base catalysts. Alkoxides react directly with silanols or with water to produce silanols. The newly formed silanols can react with other alkoxides or self-condense to produce a siloxane bond and water. When an acid catalyst is used, protonation of the alkoxysilane increases the reactivity of the leaving group. When a base catalyst is used, deprotonation of the silanol forms a reactive silonate anion. The by-product of the reaction is an alcohol. Common metal catalysts for these reactions are alkoxytitanium derivatives and dibutyltin dicarboxylates
More about: Trimethoxy(methyl)silane sale
Read more: chemical materials
CAS:1185-55-3
Molecular Formula:C4H12O3Si
Molecular Weight:136.22 g/mol
Description:
Trimethoxy(methyl)silane occurs as achromatic bright aqueous with its melting point -70°C and baking point 102-104 °C(lit.).It will decompose in water.It is stable, but damp sensitive. It is aswell awful flammable. It is adverse with water, able acids, able acerbic agents.This artefact should be stored amid 2-8°C.
Applications:
As a crosslinking agent for room temperature cured silicone rubber, coupling agent for glass fiber and SiO2, a strengthing treatment agent for plastic-layer pressing material.
The most common alkoxy crosslinkers are methoxy or ethoxy silanes due to their high reactivity. The reaction precedes by nucleophilic substitution usually in the presence of acid or base catalysts. Alkoxides react directly with silanols or with water to produce silanols. The newly formed silanols can react with other alkoxides or self-condense to produce a siloxane bond and water. When an acid catalyst is used, protonation of the alkoxysilane increases the reactivity of the leaving group. When a base catalyst is used, deprotonation of the silanol forms a reactive silonate anion. The by-product of the reaction is an alcohol. Common metal catalysts for these reactions are alkoxytitanium derivatives and dibutyltin dicarboxylates
More about: Trimethoxy(methyl)silane sale
Read more: chemical materials
Sunday, March 11, 2012
Where to get Crystal Violet Lactone?
Crystal Violet Lactone (CVL) is a leuco dye, a lactone acquired of clear violet 10B. In authentic accompaniment it is a hardly bare apparent powder, acrid in nonpolar or hardly arctic amoebic solvents.
The axial carbon in the leuco anatomy is in a tetraedric configuration, basic four covalent bonds. In acerb ambiance the lactone ring is broken, the axial carbon loses one valence and becomes a resonance counterbalanced carbocation (although it ability be bigger to draw the resonance anatomy with the cation on nitrogen), this collapsed carbon abutting the π systems of the ambrosial rings and the amino anatomic groups to anatomy one ample conjugated arrangement acting as a chromophore with able assimilation in arresting spectrum, giving this admixture its characteristic color.
Crystal Violet Lactone was the aboriginal dye acclimated in carbonless archetype papers, and it is still broadly acclimated in this application. It is aswell the leuco dye basic in some thermochromic dyes, e.g. in the Hypercolor band of clothing. One of its atypical uses is a aegis brand for fuels.
Crystal Violet Lactone may could cause allergic acquaintance dermatitis in humans administration the carbonless archetype paper.
More about: Crystal Violet Lactone sale
Read more: chemical materials
The axial carbon in the leuco anatomy is in a tetraedric configuration, basic four covalent bonds. In acerb ambiance the lactone ring is broken, the axial carbon loses one valence and becomes a resonance counterbalanced carbocation (although it ability be bigger to draw the resonance anatomy with the cation on nitrogen), this collapsed carbon abutting the π systems of the ambrosial rings and the amino anatomic groups to anatomy one ample conjugated arrangement acting as a chromophore with able assimilation in arresting spectrum, giving this admixture its characteristic color.
Crystal Violet Lactone was the aboriginal dye acclimated in carbonless archetype papers, and it is still broadly acclimated in this application. It is aswell the leuco dye basic in some thermochromic dyes, e.g. in the Hypercolor band of clothing. One of its atypical uses is a aegis brand for fuels.
Crystal Violet Lactone may could cause allergic acquaintance dermatitis in humans administration the carbonless archetype paper.
More about: Crystal Violet Lactone sale
Read more: chemical materials
What is Dicyclohexano-18-crown 6-Ether?
Description of Dicyclohexano-18-crown 6-Ether
Dicyclohexano-18-crown 6-Ether occurs as white solid with its melting point 47-50 °C and boiling point 380-344°C. Dicyclohexano-18-crown 6-Ether is slightly soluble in water.It is also hygroscopic.Its purity is not less than 98.0%(GC).Dicyclohexano-18-crown 6-Ether is the grade of EP.The specification is 1G or 5G.This product should be stored between 2-8°C.
Crown Ether is a macrocyclic polyether whose structure contains hydrogen, carbon and oxygen atoms. Each oxygen atoms are confined between two carbon atoms and exhibits a conformation with a hole (accordingly called "crown"). The common names of Crown Ethers have a prefix to designate the total number of atoms in the cycle and a suffix to designate the number of oxygen atoms in the cycle. For example, 15-crown-5 is composed of 15 atoms in the cycle, 5 of which are O and 10 of which are C.
As an ether, Dicyclohexano-18-crown 6-Ether is soluble in non-polar solvents. They are capable of strong solvency binding cations in their central cavity. The exterior of the ring is hydrophobic. The size of the interior central cavity, fused ring system, and side chains and functional groups determine the solvency capacity of the cation and the power of hydrophobic.
More about: Dicyclohexano-18-crown 6-Ether sale
Read more: chemical materials
Dicyclohexano-18-crown 6-Ether occurs as white solid with its melting point 47-50 °C and boiling point 380-344°C. Dicyclohexano-18-crown 6-Ether is slightly soluble in water.It is also hygroscopic.Its purity is not less than 98.0%(GC).Dicyclohexano-18-crown 6-Ether is the grade of EP.The specification is 1G or 5G.This product should be stored between 2-8°C.
Crown Ether is a macrocyclic polyether whose structure contains hydrogen, carbon and oxygen atoms. Each oxygen atoms are confined between two carbon atoms and exhibits a conformation with a hole (accordingly called "crown"). The common names of Crown Ethers have a prefix to designate the total number of atoms in the cycle and a suffix to designate the number of oxygen atoms in the cycle. For example, 15-crown-5 is composed of 15 atoms in the cycle, 5 of which are O and 10 of which are C.
As an ether, Dicyclohexano-18-crown 6-Ether is soluble in non-polar solvents. They are capable of strong solvency binding cations in their central cavity. The exterior of the ring is hydrophobic. The size of the interior central cavity, fused ring system, and side chains and functional groups determine the solvency capacity of the cation and the power of hydrophobic.
More about: Dicyclohexano-18-crown 6-Ether sale
Read more: chemical materials
Thursday, March 8, 2012
Descriptions of 4,4-(2-Ethylhexylidene)diphenol
4,4-(2-Ethylhexylidene)diphenol
CAS:74462-02-5
Molecular Formula:C20H26O2
Molecular Weight: 298.42g/mol
Description of 4,4-(2-Ethylhexylidene)diphenol
The melting point of 4,4-(2-Ethylhexylidene)diphenol is 87 °C. Its grade is EP. The specification is 25G
More about: 4,4-(2-Ethylhexylidene)diphenol sale
Read more: chemical materials
CAS:74462-02-5
Molecular Formula:C20H26O2
Molecular Weight: 298.42g/mol
Description of 4,4-(2-Ethylhexylidene)diphenol
The melting point of 4,4-(2-Ethylhexylidene)diphenol is 87 °C. Its grade is EP. The specification is 25G
More about: 4,4-(2-Ethylhexylidene)diphenol sale
Read more: chemical materials
Wednesday, March 7, 2012
Features of Extra Fine Silver Powder
Features of Extra Fine Silver Powder
The silicon metal is Silver gray or dark grey powder, Heat-resistance and good performance.
Application
Extra Fine Silver Powder possesses quite widely. For example, to produce organic-Si(Si-oil, Si-rubber) etc in chemical industry. And also regarding the no-ferroalloy’s additive, deoxidize, silicon-steel etc alloys adding materials. It’s applied to mechanical, chemical, alloys, electronic, medicine industry etc.
Extra Fine Silver Powder's widely used in the fields of electric conductive and magnetic conductive materials, high performance electronic pulp, high efficient catalyst and antibiotic reagent.
More about: Extra Fine Silver Powder sale
Read more: Chemical Compound
Specifications of O-Acetylricinoleic Acid Methyl Ester
Description of O-Acetylricinoleic Acid Methyl Ester
O-Acetylricinoleic Acid Methyl Ester is also called methyl (Z)-12-acetyloxyoctadec-9-enoate. CAS is 140-03-4. Molecular Formula is C21H38O4. Molecular Weight is 354.52g/mol.
The melting point of O-Acetylricinoleic Acid Methyl Ester is -26°C and baking point is 185°C at 2mmHg. Its abstention is not beneath than 80.0%(GC). The blueprint is 25ML or 500ML.
More about: O-Acetylricinoleic Acid Methyl Ester Sale
Read more: chemical materials
O-Acetylricinoleic Acid Methyl Ester is also called methyl (Z)-12-acetyloxyoctadec-9-enoate. CAS is 140-03-4. Molecular Formula is C21H38O4. Molecular Weight is 354.52g/mol.
The melting point of O-Acetylricinoleic Acid Methyl Ester is -26°C and baking point is 185°C at 2mmHg. Its abstention is not beneath than 80.0%(GC). The blueprint is 25ML or 500ML.
More about: O-Acetylricinoleic Acid Methyl Ester Sale
Read more: chemical materials
Monday, March 5, 2012
Definitions of Tributyrin
Tributyrin, also known as Butyrin, is a triglyceride naturally present in butter. It is an ester composed of butyric acid and glycerol. Among other things, it is used as an ingredient in making margarine. It is commonly occurring in butter and can be described as a liquid fat with an acrid taste.
Tributyrin is also used in microbiological laboratories to identify the bacterium Moraxella catarrhalis.
Tributyrin is a stable and rapidly absorbed prodrug of butyric acid which enhances antiproliferative effects of dihydroxycholecalciferol in human colon cancer cells.
Tributyrin has broad anti-cancer actions and synergizes many therapies. This approved food additive also normalizes mitochondrial membrane potential.
Tributyrin, a triglyceride analogue of butyric acid (butyryl triglyceride), is similar in structure to 95% of the fatty acids found in foods, can be p.o.(orally) administered, is well tolerated even by young children, and is an approved food additive.
More about: Tributyrin sale
Read more: chemical materials
Tributyrin is also used in microbiological laboratories to identify the bacterium Moraxella catarrhalis.
Tributyrin is a stable and rapidly absorbed prodrug of butyric acid which enhances antiproliferative effects of dihydroxycholecalciferol in human colon cancer cells.
Tributyrin has broad anti-cancer actions and synergizes many therapies. This approved food additive also normalizes mitochondrial membrane potential.
Tributyrin, a triglyceride analogue of butyric acid (butyryl triglyceride), is similar in structure to 95% of the fatty acids found in foods, can be p.o.(orally) administered, is well tolerated even by young children, and is an approved food additive.
More about: Tributyrin sale
Read more: chemical materials
What is Terephthaloyl Chloride?
Terephthaloyl Chloride (TCL, 1,4-benzenedicarbonyl chloride) is the acid chloride of terephthalic acid and is one of two monomers used to make Kevlar, the other being p-phenylenediamine. Its CAS reference number is 100-20-9 and its chemical formula is C8H4Cl2O2.
TCL is used as a key component in performance polymers and aramid fibers, where it imparts flame resistance, chemical resistance, temperature stability, light weight, and very high strength. TCL is also an effective water scavenger, used to stabilize isocyanates and urethane prepolymers.
Terephthaloyl Chloride is a white crystalline solid at room temperature, soluble in common organic solvents. Its melting point is 81.5-83 °C, its boiling point is 265 °C. It is corrosive.
TCL is used for making various copolymers and aramid polymers such as Twaron and kevlar
More about: Terephthaloyl Chloride sale
Read more: chemical materials
TCL is used as a key component in performance polymers and aramid fibers, where it imparts flame resistance, chemical resistance, temperature stability, light weight, and very high strength. TCL is also an effective water scavenger, used to stabilize isocyanates and urethane prepolymers.
Terephthaloyl Chloride is a white crystalline solid at room temperature, soluble in common organic solvents. Its melting point is 81.5-83 °C, its boiling point is 265 °C. It is corrosive.
TCL is used for making various copolymers and aramid polymers such as Twaron and kevlar
More about: Terephthaloyl Chloride sale
Read more: chemical materials
Sunday, March 4, 2012
What is 2-Nitrobiphenyl?
2-Nitrobiphenyl
CAS:86-00-0
Molecular Formula:C12H9NO2
Molecular Weight: 199.21g/mol
vapor density 5.9 (vs air)
vapor pressure 2 mmHg ( 140 °C)
assay 97%
autoignition temp. 356 °F
bp 165-170 °C/13 mmHg(lit.)
mp 36-38 °C(lit.)
2-Nitrobiphenyl occurs as gold to tan crystals or brown solid with its melting point 36-38 °C(lit.) and boiling point 320 °C. 2-Nitrobiphenyl is insoluble in water. Its purity is not less than 98.0%(GC). 2-Nitrobiphenyl's the grade of EP.The specification is 25G or 500G.
More about: 2-Nitrobiphenyl sale
Read more: chemical materials
CAS:86-00-0
Molecular Formula:C12H9NO2
Molecular Weight: 199.21g/mol
vapor density 5.9 (vs air)
vapor pressure 2 mmHg ( 140 °C)
assay 97%
autoignition temp. 356 °F
bp 165-170 °C/13 mmHg(lit.)
mp 36-38 °C(lit.)
2-Nitrobiphenyl occurs as gold to tan crystals or brown solid with its melting point 36-38 °C(lit.) and boiling point 320 °C. 2-Nitrobiphenyl is insoluble in water. Its purity is not less than 98.0%(GC). 2-Nitrobiphenyl's the grade of EP.The specification is 25G or 500G.
More about: 2-Nitrobiphenyl sale
Read more: chemical materials
Descriptions of TCPP (Tetrakis(4-carboxyphenyl)porphyrin Ultra-high sensitive spectrophotometric reagent for Cu, Cd For the simultaneous determination of metals by HPLC)
TCPP (Tetrakis(4-carboxyphenyl)porphyrin Ultra-high sensitive spectrophotometric reagent for Cu, Cd For the simultaneous determination of metals by HPLC)
CAS:14609-54-2
Molecular Formula:C48H30N4O8
Molecular Weight: 790.77g/mol
Description of TCPP (Tetrakis(4-carboxyphenyl)porphyrin Ultra-high sensitive spectrophotometric reagent for Cu, Cd For the simultaneous determination of metals by HPLC)
TCPP (Tetrakis(4-carboxyphenyl)porphyrin Ultra-high sensitive spectrophotometric reagent for Cu, Cd For the simultaneous determination of metals by HPLC)'s purity is not less than 97.0%(LC)(T).Its grade is Ace.Its specification is 100mg or 1g.
Synonyms
Mtcpp;Aids006254;Aids-006254;NICKEL IONOPHORE II;RARECHEM AS SA 0048;tetracarboxyphenylporphine;TETRACARBOXYPHENYLPORPHYRIN;Tetrakis(carboxphenyl)porphyrin;TETRAKIS(4-CARBOXYPHENYL)PORPHINE;Tetrakis(4-carboxyphenyl)porphyrin
More about: TCPP (Tetrakis(4-carboxyphenyl)porphyrin Ultra-high sensitive spectrophotometric reagent for Cu, Cd For the simultaneous determination of metals by HPLC) sale
Read more: chemical materials
CAS:14609-54-2
Molecular Formula:C48H30N4O8
Molecular Weight: 790.77g/mol
Description of TCPP (Tetrakis(4-carboxyphenyl)porphyrin Ultra-high sensitive spectrophotometric reagent for Cu, Cd For the simultaneous determination of metals by HPLC)
TCPP (Tetrakis(4-carboxyphenyl)porphyrin Ultra-high sensitive spectrophotometric reagent for Cu, Cd For the simultaneous determination of metals by HPLC)'s purity is not less than 97.0%(LC)(T).Its grade is Ace.Its specification is 100mg or 1g.
Synonyms
Mtcpp;Aids006254;Aids-006254;NICKEL IONOPHORE II;RARECHEM AS SA 0048;tetracarboxyphenylporphine;TETRACARBOXYPHENYLPORPHYRIN;Tetrakis(carboxphenyl)porphyrin;TETRAKIS(4-CARBOXYPHENYL)PORPHINE;Tetrakis(4-carboxyphenyl)porphyrin
More about: TCPP (Tetrakis(4-carboxyphenyl)porphyrin Ultra-high sensitive spectrophotometric reagent for Cu, Cd For the simultaneous determination of metals by HPLC) sale
Read more: chemical materials
Thursday, March 1, 2012
Functions of Laccaic Acid from Lacca
Description
Laccaic Acid from Lacca is a red colored natural dye produced by the insect Laccifer lacca or Coccus lacca. It is obtained in large amounts as a by-product of the shellac industry and has been considered for general use as a food coloring agent. Laccaic acid is found to have no mutagenic activity as assessed by two short-term assays: the Salmonella/microsome mutagenicity test, and the ØX fidelity assay. However, laccaic acid did inhibit metabolic cooperation in Chinese hamster V79 cells. These results suggest that laccaic acid should be tested in animals with particular emphasis on in vivo models for tumor promotion.
Function of Laccaic Acid from Lacca
With the function of natural food red color pigment, it is widely used in baked food, beverages of fruit vegetable juice (pulp), fruit flavoured beverages,soda pop, compound seasonings, jam, ice cream, ham, sausage,chocolate and candy products, it has good coloring effect
Application:
Applied in food field, it is mainly used as natural food additives for pigment
Applied in cosmetic field and pharmaceuticals , it is mainly used as red pigment
As senior dye, widely used in textile manufacturing industry, such as toys, senior textile, wool products and more
Storage: store in cool and dry places, keep away from light
More about: Laccaic Acid from Lacca sale
Read more: chemical materials
Laccaic Acid from Lacca is a red colored natural dye produced by the insect Laccifer lacca or Coccus lacca. It is obtained in large amounts as a by-product of the shellac industry and has been considered for general use as a food coloring agent. Laccaic acid is found to have no mutagenic activity as assessed by two short-term assays: the Salmonella/microsome mutagenicity test, and the ØX fidelity assay. However, laccaic acid did inhibit metabolic cooperation in Chinese hamster V79 cells. These results suggest that laccaic acid should be tested in animals with particular emphasis on in vivo models for tumor promotion.
Function of Laccaic Acid from Lacca
With the function of natural food red color pigment, it is widely used in baked food, beverages of fruit vegetable juice (pulp), fruit flavoured beverages,soda pop, compound seasonings, jam, ice cream, ham, sausage,chocolate and candy products, it has good coloring effect
Application:
Applied in food field, it is mainly used as natural food additives for pigment
Applied in cosmetic field and pharmaceuticals , it is mainly used as red pigment
As senior dye, widely used in textile manufacturing industry, such as toys, senior textile, wool products and more
Storage: store in cool and dry places, keep away from light
More about: Laccaic Acid from Lacca sale
Read more: chemical materials
Subscribe to:
Comments (Atom)








silane.gif)










silane.gif)